Wild-type ATTR is also referred to as ATTRwt. It is not caused by any known genetic mutations, such as in the case of hereditary forms of the disease (hATTR). This disease used to be called SSA or SCA, which stood for Senile Systemic Amyloidosis and Senile Cardiac Amyloidosis, respectively, which are now outdated terminologies. The disease is not known to be directly related to dementia, but it is related to aging.
Deposits of TTR amyloid can be found throughout the body, so it is a systemic amyloidosis disease. The most common place it is found is in the heart. Wild-type ATTR is also known to cause some cases of carpal tunnel syndrome, which can be the first (early) symptom. Recent data suggests that lumbar spine involvement as well as a rupture of the biceps tendon in the forearm can precede cardiac involvement by many years.
This is a disease that has traditionally been found mostly in men, originally reported in those aged 80 and over. As awareness of the disease increases, wild-type ATTR average age at diagnosis is 75. It is often overlooked as an amyloidosis disease because so many people experience heart problems in their later years.
As with hereditary forms of the disease (hATTR) wild-type ATTR causes problems due to the breaking apart, misfolding and deposition of amyloid protein fibrils in healthy tissue. “Wild-type” refers to this form of the disease because it is the natural form of this protein, without genetic mutation. These deposits can interfere with the heart’s normal function, by causing stiffness of the heart tissue, making it more difficult for the heart to fill, leading to heart rhythm problems and heart failure.
- Symptoms
Symptoms
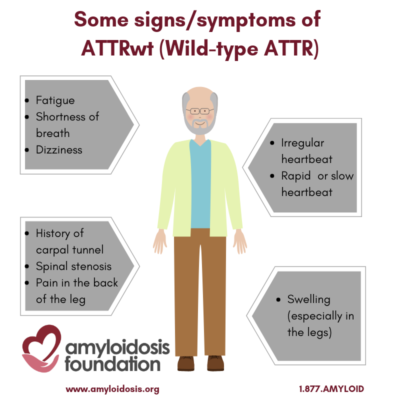
Amyloid deposits in the heart can make the heart unable to function efficiently because it does not fill easily and freely, as it should. This may result in shortness of breath, dizziness, fatigue or edema (swelling, especially in the legs). Some symptoms may occur with only minor activity.
Since the stiffened heart fills less with each heartbeat, the heart rate speeds up, to deliver more blood to the brain. One of the common treatments for other forms of Congestive Heart Failure is to slow down the heartrate. This is not helpful for people with ATTR. It may block the heart from catching up to the brain’s demand for more oxygen.
Another effect of the stiffened heart is that the atria, or upper chambers, become expanded. The swelling and expansion of these chambers may stretch the wall where the electrical system is contained, causing a rapid irregular heartbeat, known as atrial fibrillation.
For some older men, a history of carpal tunnel syndrome (especially without a clear cause), along with heart problems, is a signal to the doctor to consider testing for wild-type ATTR. Orthopedic manifestations also include lumbar spinal stenosis and rupture of a biceps tendon. To a lesser extent, wild-type ATTR has shown amyloid deposits in the lungs, bladder, and bowel, often with no, or minor, symptoms for the patient; although some patients have extensive bladder involvement that can lead to hematuria (blood in the urine).
In addition, peripheral neuropathy symptoms are present in a small number of patients, and lumbar spinal stenosis has also been found to be related to wild-type ATTR. Spinal stenosis narrows the spaces in the backbone and puts pressure on the spinal cord and nerves. Symptoms may be caused by the pressure on the nerve root, causing pain.
- Diagnosis
Diagnosis
ATTR can cause very similar symptoms to other forms of Congestive Heart Failure, but those other forms of Congestive Heart Failure are treated with medications that can be harmful to people with ATTR. On the other hand, a patient with AL (light chain) Amyloidosis who shows cardiomyopathy symptoms will often be treated with chemotherapy – and this treatment is harmful to wild-type ATTR patients with cardiomyopathy. For these reasons, the correct and full diagnosis of ATTR is necessary and critical.
In the past, it was difficult to get a positive diagnosis of wild-type ATTR. If ATTR-wt was suspected a biopsy of the heart tissue was needed to get an accurate diagnosis. However, studies using nuclear scintigraphy with agents used to evaluate bones have shown that they can diagnose wild-type and hereditary TTR cardiac amyloidosis without the need for a biopsy. These scans need to be coupled with blood tests to evaluate for the substrate for AL amyloid (e.g. light chains) and in the absence of any evidence of light chains and a positive scan, are able to diagnose ATTR cardiac amyloidosis. However, if there is evidence of light chains on blood testing, then a biopsy is still needed to establish the diagnosis and type of amyloidosis.
If a biopsy of the heart tissue is obtained, then this biopsy tissue is sent to a lab for Congo-red staining. The lab will stain the biopsy and, if it turns an apple green color under a ‘polarizing’ microscope, then amyloidosis is confirmed. The lab will also take the biopsy tissue and run a protein sequence analysis test to see which type of protein is affected. If this test shows that the transthyretin (TTR) protein is involved, then a simple blood sample is sent to a lab and experts do a genetic sequencing test to examine the DNA chains.
If this TTR genetic sequencing test produces no identifiable mutations, then wild-type ATTR is the resulting diagnosis. So you can see that it takes several steps and a doctor must continue testing until an accurate diagnosis is achieved.
- Monitoring
Monitoring
Blood tests used to detect stress and strain on the heart tissue are useful in monitoring wild-type ATTR. These cardiac biomarkers include troponin T or troponin I, and NT-proBNP (which stands for N-terminal pro-brain natriuretic peptide) or BNP (brain natriuretic peptide). Different laboratories use one versus the other.
The echocardiogram (also called echo”) is an ultrasound of the heart. A doctor can look at the size and shape of the heart, and whether it is relaxing normally in between heartbeats. Amyloid cannot be seen directly, but it does make the heart larger and stiffer than normal. The thickness of the heart walls and the measures of heart stiffness may be signs of the disease’s progress, or stability.
Other imaging tests for the heart have also been shown to be useful. One test is the MRI (magnetic resonance imaging), and, in this instance, it is also referred to as CMR (for cardiac magnetic resonance).
- Treatment
Treatment
(FDA) has approved both VYNDAQEL® (tafamidis meglumine) and VYNDAMAX™ (tafamidis) for the treatment of the cardiomyopathy of wild-type or hereditary transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular mortality and cardiovascular-related hospitalization. VYNDAQEL and VYNDAMAX are two oral formulations of the first-in-class transthyretin stabilizer tafamidis,
It is important to understand that there are some treatments that are used to manage the symptoms of ATTR, and other treatments that are used to modify the disease progress of ATTR.
Typically, for congestive heart failure problems, diuretics are prescribed to increase urination, which helps to decrease fluid retention in the body. The same is true for ATTR. They help reduce swelling and shortness of breath. As with any other condition, the use of diuretics for an amyloid heart condition must be carefully controlled by your doctor. All diuretic dosing can be helped by limiting the amount of sodium that you consume.
For other forms of Congestive Heart Failure, beta-blockers, ACE inhibitors, and Calcium channel blockers are given to increase the pumping power of the heart, but these drugs can be harmful to people with ATTR.
It should be noted that there are several clinical trials that are in progress to deal with ATTRwt. The open trials can be found at clinicaltrials.gov
- FAQs
FAQs
What does TTR mean?
Since systemic amyloidoses are referred to with a capital A (for amyloid) followed by an abbreviation for the fibril protein, ATTR amyloidosis stands for the protein transthyretin (TTR); so these diseases are often designated with the acronym ATTR.
Note that “wild-type ATTR” and “hATTR” are two different diseases. Wild-type ATTR is not hereditary, while hATTR is hereditary. However, both diseases involve “TTR,” which stands for “transthyretin.”
In medical texts, transthyretin (formerly called prealbumin) is defined as a normal protein in the blood. In simpler terms, transthyretin helps to move the thyroid hormone and vitamin A (retinol) in your body. Thus, the name Transthyretin, which means that it TRANSports THYroxine and RETINol.
What is the difference between Familial ATTR and Wild-type ATTR?
Transthyretin (TTR) has subunits in the TTR blood protein that can produce two forms of systemic amyloidosis: they are the mutant TTR and wild-type TTR amyloid diseases.
Familial ATTR (or hATTR)– Mutant TTR: This is the hereditary form and is also referred to as the ‘mutant’ form of TTR amyloid diseases because the protein sequence it contains is abnormal given the presence of a mutation. This results in an unstable protein that is prone to misfold as a result of mutations in a patient’s inherited genetic code.
Wild-type ATTR (or ATTRwt) – Normal TTR: This is the non-hereditary form and is often referred to as the ‘normal’ TTR amyloid disease because it does not exist as a result of genetic mutations. Wild-type ATTR is a collection of misfolded amyloid proteins that travel into the organs and are only from the normal wild-type transthyretin.
The regular function of both of these subunits of TTR is to carry the thyroid hormone and vitamin A (retinol) within the bloodstream.
What causes Wild-type ATTR?
Wild-type ATTR is not considered to be a hereditary disease. The exact mechanism of how normal or wild type transthyretin results in amyloidosis is not yet definitively determined but the prevailing opinion is that “normal” wild-type misfolds and deposits in the heart and other affected tissues.
Even though it is not considered to run in families, it is not known if this disease is caused by an “epigenetic” factor. Epigenetic means that someone can inherit changes in their gene function, but it does not involve changes in their DNA sequence. Research continues in this area.
Wild-type ATTR is not contagious.
How common is Wild-type ATTR Amyloidosis?
Medical statistics vary, but it is thought that wild-type ATTR may be present in around 1% of males over 80 years of age. How much amyloid deposition is necessary to cause symptoms such as heart failure is not known. However, it is also believed that only 25% of this same age group may experience symptoms.
ATTRwt is underdiagnosed and thus may not be as rare as the statistics show. These are just some of the reasons that exist for this theory:
- Heart problems are common in older patients. In order to accurately diagnose the patient, a series of cardiac tests may be necessary. These test are not always performed, especially if there is a financial concern and/or a lack of insurance coverage. An attitude that such problems are inevitable also lessens the motivation of some to investigate further.
- Since wild-type ATTR is thought to be rare, it is not considered as a potential cause of common cardiovascular conditions such as heart failure with a preserved ejection fraction. There may be a fear of complications from a heart biopsy with older patients. In the past if a biopsy were not performed, it would result in an incomplete diagnosis. Now a PYP scan can be done with much less risk, and result in a complete diagnosis, which would hopefully change the nature and outcome of its treatment.
More awareness of this disease within the medical community, and the public at large, is needed.
What are the symptoms of heart involvement with wild-type ATTR?
When amyloid deposits cause cardiomyopathy in wild-type ATTR, it can result in a stiffening of the heart. Some patients may experience:
- Shortness of breath
- Increasing fatigue
- Weight loss
- Inability to sleep
- Dizziness
- Nausea
Shortness of breath, leg swelling, fatigue and atrial fibrillation are the most common symptoms. The term “arrhythmia” refers to changes in the normal electrical impulses that cause the heart to beat. The result is a heart that can beat too fast, too slow or erratically. Atrial fibrillation (or a-fib for short) is one of many forms of arrhythmia. During a-fib, the heart’s two small upper chambers cause an abnormal heart rhythm, usually rapid and irregular beating. This may result in increased heart damage, stroke or heart failure.
How do they diagnose the TYPE of amyloidosis?
TTR cardiac amyloidosis can now be diagnosed without a biopsy. A combination of “bone” scan or nuclear scintigraphy coupled with blood tests can be used to evaluate for proteins associated with the AL or light chain type of amyloidosis. If the nuclear scan is positive and there is no evidence of any monoclonal proteins, then the diagnosis of TTR cardiac amyloidosis can be made with certainty.
For patients who have blood tests that show a monoclonal protein, then a biopsy is required for definitive diagnosis. If a biopsy shows amyloid, then identifying the type of amyloid protein is the next crucial step. This is usually done by evaluating the tissue further with a technique called mass spectroscopy to determine which protein is causing the amyloid deposition. Treatments can differ and should be tailored to the patient and the exact type of amyloidosis that they have.
Typing can be done by a variety of lab techniques, including:
-
Proteomics (the study of proteins) by mass spectrometry. Lasers are used to cut biopsy tissue samples into very small sections. These sections can then be broken down further for analysis. The mass spectrometer separates a small biopsy section turning it into beams of particles and bending it with electricity and magnetics to make a kind of spectrum, which allow the molecules to be counted and the amyloid to chemically tested, so the exact type of amyloid protein can be identified. This technique is usually very precise, however, it is not available in all labs and is currently an expensive testing technique.
-
Immunohistochemistry is a common and more affordable testing technique that is used to identify the types of proteins in tissues by the use of markers, like fluorescent dyes or enzymes. The biopsy sample is viewed with electron microscopy, which magnifies very small details using a beam of electrons to separate the elements. Or, it is viewed with light microscopy, which uses light wavelengths to probe and separate the sample. Immunohistochemistry works well for wild-type ATTR heart biopsies, however it still needs to be coupled with DNA analysis to confirm the identity of wild-type ATTR versus hereditary ATTR.
In all cases, after the diagnosis of TTR amyloidosis is confirmed, further DNA analysis should be performed to differentiate wild-type ATTR from hereditary ATTR amyloidosis. hATTR is inherited in an autosomal dominant fashion (meaning that any first degree relative of an affected individual has a 50/50 chance of inheriting the abnormal gene.
The accuracy of each form of typing depends upon the technique itself, but also on the ability and experience of the laboratory performing them. It is best to have typing done in a reference laboratory at an experienced center, and the typing should agree with the clinical features of the patient’s disease. Unfortunately, costs of typing are not always covered by insurance, but it is very important to have correct typing before any treatment is initiated.
What heart tests are helpful for the monitoring of wild-type ATTR?
If heart involvement is suspected, then blood tests for heart biomarkers can show signs of heart tissue strain or damage in a person’s blood. The results of these tests can be used as “markers” (or “biomarkers”) to first determine the extent of any damage, and then can be used regularly to monitor any future problems. Two troponin tests can be done — cardiac serum troponin T and cardiac serum troponin I. The other important biomarker is N-terminal pro-brain natriuretic peptide (NT-proBNP) or brain natriuretic peptide (BNP). Different laboratories use one versus another.
- Cardiac troponins T (cTnT) and I (cTnI) are released when the heart muscle has had some injury. In general, the more damage there is to the heart, the greater the amount of troponin T and I there will be in the blood.
- NT-proBNP is another “biomarker” that should be performed, especially if a person has symptoms such as swelling in the legs (edema), difficulty breathing, shortness of breath, and fatigue. It is used to detect heart stress or strain. This blood test can be useful in distinguishing fluid in the lungs due to congestive heart failure (in which it would be elevated) or from lung or pleural disease (in which case it should be normal or near normal).
- These biomarker blood tests can be affected by changes in kidney function, drugs, and other causes. They should be interpreted in the context of other tests of cardiac function, such as an echocardiogram or cardiac magnetic resonance imaging.
The echocardiogram (also called “echo”) is an ultrasound of the heart. A doctor can look at the size and shape of the heart, and whether it is relaxing normally in between heartbeats. Amyloid cannot be seen directly, but it does make the heart larger and stiffer than normal. The thickness of the heart walls and the measures of heart stiffness are believed to be signs of the disease’s progress, or stability.
Other imaging tests for the heart have also been shown to be useful. One test is the MRI (magnetic resonance imaging), and, in this instance, is also referred to as CMR (for cardiac magnetic resonance). CMR with a contrast agent called “gadolinium”, given by vein at the time of the scan, is a way to detect amyloid deposits in the heart.
Nuclear scintigraphy with either PYP, DPD or HMDP (three isotopes that have been used to image bones for decades) can diagnose TTR cardiac amyloidosis without the need for a biopsy. An intravenous injection of isotope (PYP, DPD or HMDP) is delivered while the patient is at rest, followed one to three hours later by a set of images that are taken while the patient lies down under a camera. The images take about 10 minutes. These images are recorded on a computer for analysis, and recent data suggests this scan may be useful in distinguishing different types of amyloid heart disease.
What is the difference between DNA and RNA?
In simple terms, our DNA stores and transfers genetic information. A gene tells a cell how to make a specific protein.
Proteins are formed inside our cells and it is our DNA that holds the “recipe” for making proteins. DNA and RNA work together and they both carry genetic information to make up the many different proteins we need. However, they perform different functions for this task.
The RNA helps to move the DNA “code” from storage to where it can be used. RNA is converted (or “translated”) into a sequence of amino acids that makes up a protein.
The small interfering RNA (siRNA) are natural quality control engineers in the body’s protein production facility, and they monitor the proteins produced, squelching incorrectly folded or otherwise faulty proteins.
In basic biological terms: Transcription = DNA → RNA Translation = RNA → protein à siRNA.
The collections of proteins within a cell are essential for our body’s health and function, and they work in a variety of ways, serving actively inside the cell as well as interacting outside the cell – in virtually every process within the body.
What kind of doctor should be consulted?
It is strongly recommended that you consult with a specialist in the field of amyloidosis. The Amyloidosis Foundation provides a list of amyloidosis treatment centers under “Patient Resources” on this website. Once your diagnosis is confirmed, then a treatment plan can be laid out for your individual case. Depending on your symptoms, you will probably be seeing a local neurologist (nerves), cardiologist (heart), gastroenterologist (GI tract), internist and/or general physician. These doctors should coordinate your care with the amyloidosis specialist to develop the best treatment program.
Is there a special diet that I can follow?
Eating a well-balanced and nutritious diet is always recommended. However, in cases of ATTR, the most important component of your diet is to limit your intake of sodium. The more sodium that you consume, the more likely it is that your blood will retain fluid, in order to counter act the elevated sodium eaten.
The higher the amount of fluid retained in your blood, the more difficult it is for your already stiffened heart to manage that increased blood volume.
Increases in salt intake lead directly to increases in swelling, shortness of breath and fatigue. Elevated salt intake also requires greater doses of diuretics, which lead to greater kidney burden, and hasten the harm to your kidneys which is already present due to necessary diuresis.
Although amyloid is an abnormal protein, the amount of protein in the diet does not affect the onset of the disease. Remember that ATTR is caused by the protein that we make, not the protein that we take.
Consult with your physician on any dietary changes, and report any vitamins or other supplements that you take. You are a part of the team of people who must keep in communication with each other about your health.
Updated March 2021
