There is a wide spectrum of amyloidosis diseases, and the most common of them have been addressed in other areas on this website. All forms of amyloidosis have this in common: an overabundance of abnormal protein production. These misfolded amyloid proteins are poorly structured causing them to gather together and deposit into areas of the body. As this deposition increases it can disrupt the normal function of, and cause damage to, one organ or tissue or multiple organs and tissues.
Determining the type of protein is the key. Depending on the type of amyloidosis, the treatment and recovery varies. It is critically important for the physician to accurately type the amyloid protein in order to follow an appropriate treatment protocol.
The Amyloidosis Foundation directs the focus to some other, less common, amyloid protein types in this section. If you find that you are diagnosed with a type that has limited material available, please contact us and we will provide you with information and referrals to amyloidosis specialists to help you better understand your situation.
- ALECT2
ALECT2
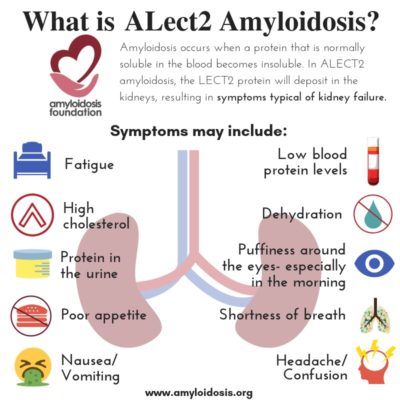
Leukocyte chemotactic factor 2 (LECT2) is a protein that is made in the liver, circulates in the blood, and its complete function in the body is still undetermined. Scientists believe that it may be part of cartilage reconstruction and tissue repair, autoimmune response, or it may be part of our cell growth, among other possibilities.
While there still exists a possibility that ALECT2 has some hereditary and genetic connection, the classification of this disease is currently listed as an ‘acquired’ systemic amyloidosis disease, and not a hereditary one. This is because there is no one genetic mutation found in any patient that has been diagnosed with ALECT2. However, the scientists question if ALECT2 is a “digenic disease.” “Di” means “two” in this case, which means that it could be the result of a combination of two disorders in the genetic sequence, involving a genetic mutation that has not yet been identified.
With the overproduction of this normal protein triggering misfolded amyloid proteins, the unknown cause for this overproduction means that there is no absolute conclusion in the classification for ALECT2 at this time. Research continues in this area.
Are there symptoms for ALECT2?
ALECT2 organ involvement is mainly associated with kidney damage. Recent studies show that liver involvement with ALECT2 could also be more common than previously thought. However, even though amyloid protein has been discovered in liver specimens, most patients have not presented with symptoms from liver damage. Along with kidney damage and liver involvement, there have been reports of findings of amyloid in the spleen, colon, and adrenal glands in a number of ALECT2 patients, although symptoms were also not often evident in those areas.
Many experts agree that ALECT2 amyloidosis should be considered while looking for a diagnosis when a patient has evidence of renal (kidney) disease. A patient’s symptoms may appear as renal failure or “nephrotic syndrome.” Nephrotic syndrome is a group of symptoms that relate to kidney problems, including: protein found in the urine, high cholesterol levels, low blood protein levels, and swelling.
Although there is still little known about ALECT2 when compared to other forms of amyloidosis diseases, it is extremely important to get an accurate diagnosis of ALECT2 and not mistake it for the more common AL (or any other) amyloidosis disease that may cause kidney damage. With AL and so many of the amyloidosis diseases, the treatments vary one from another and can be harmful to the patient if a certain treatment is prescribed for an amyloidosis disease that is not accurately diagnosed.
How is ALECT2 diagnosed?
Like all amyloidosis diseases, the diagnosis of amyloidosis is based on the evidence of amyloid deposits and a defined organ involvement. When renal damage occurs, it is clinically shown either as proteinuria (excess protein found in the urine), nephrotic syndrome, or impairment of renal (kidney) function.
A test involving a 24-hour urine collection should be performed to look at the level of protein in the patient’s urine sample. Protein in the urine is an indication of kidney involvement. If amyloidosis is suspected through this and other test results and associated symptoms, in most cases a biopsy of renal (kidney) tissue is recommended to get an accurate diagnosis.
This renal biopsy tissue is sent to a lab for Congo-red staining. The lab will stain the biopsy. If it turns an apple green color under a ‘polarizing’ microscope then amyloidosis is confirmed. Once this initial diagnosis has been determined, the type of amyloid protein must be identified.
Typing can be done by a variety of lab techniques, including:
1. Proteomics (the study of proteins) by mass spectrometry. LMDMS = “Laser microdissection/mass spectrometer.” LMD (“laser microdissection”) uses lasers for a contact and contamination-free way to cut biopsy tissue samples into very small sections. These sections can then be broken down further for analysis. The MS (“mass spectrometer”) is a device that separates a small biopsy section through a multi-step process, turning them into beams of particles and bending them with electricity and magnetics to make a kind of spectrum. Then the device can tally and identify the molecules in that tissue sample. This allows the amyloid to be further broken down and chemically tested, so the exact type of amyloid protein can be identified. This technique is usually the most helpful for diagnosing ALECT2, however, it is not available in all labs and is currently an expensive testing technique. Specialists recommend using LMDMS if using “Immunohistochemistry” (see #2 below) does not give clear and accurate results and if the diagnosis is still in question.
2. Immunohistochemistry is a common and more affordable testing technique that is used to identify the types of proteins in tissues by the use of markers like fluorescent dyes or enzymes. In order to identify the amyloid type, the lab specialist stains the tissue sample with antibodies that are specific for the major known amyloid protein diseases. The biopsy sample can also be viewed with microscopy, which magnifies very small details and uses a beam of electrons to separate the elements. This lab technique does not always deliver a conclusive diagnosis for ALECT2.
The accuracy of each form of typing depends not only on the technique itself but also on the ability and experience of the laboratory performing them. It is best to have typing done in a reference laboratory at an experienced amyloidosis center. The typing should agree with the clinical features and symptoms of the patient’s disease. Unfortunately, the costs of typing are not always covered by insurance, but it is very important to have correct typing before any treatment is initiated.
How common is ALECT2?
ALECT2 amyloidosis has been found in a very high number in the Hispanic population; as much as 90% of the Lect2 amyloid cases in some areas. Diagnosis seems to be higher in patients that are in certain geographic (and ethnic) areas. A very small percentage has been identified in Native American patients, and even smaller percentages have been diagnosed in Caucasian, Arab, Israeli and Sudanese patients.
As more patients are diagnosed, researchers are finding that it has been an amyloidosis disease that has been vastly underdiagnosed and is fairly common in the Southwestern region of the United States. For example, in one case study, 54% of the diagnosed amyloidosis diseases were ALECT2 in the Southwestern region (including Arizona, New Mexico, and West Texas).
Over 30 proteins exist that form amyloid in humans. In the U.S., the most common type is AL (light chain) amyloidosis. Other types include AA, Wild-type ATTR, Hereditary ATTR, and Non-TTR amyloidosis diseases. Statistics vary, however, and research may prove that ALECT2 is the second or third most common amyloidosis disease in the U.S.
What is the age range of a patient with ALECT2?
Current research shows that patients have been diagnosed as young as 43 years of age and up to 88 years of age. The average age of patients is around 66 years old.
Treatment Options for ALECT2
There is no specific treatment for ALECT2 at this time. If a patient’s kidney function is compromised then it is recommended that they be seen by a nephrologist who specializes in amyloidosis because of the complexity of the disease. Regular therapy may include the maintenance of body fluid and the use of diuretics (water tablets) which help the body to lose excess salt and water. The patient may be asked to avoid excess fluid intake. In addition, regular blood and urine tests are recommended to monitor the patient’s renal function.
If kidney damage becomes severe or results in renal failure, dialysis is necessary. It is still unknown if renal transplantation is a viable option for all patients with ALECT2 and is considered on a case-by-case basis. These cases will continue to be documented to determine the success rates over time.
What kind of doctor should be consulted?
It is strongly recommended that you consult with a specialist in the field of amyloidosis. This is especially true with ALECT2, a newly discovered type of amyloidosis disease. The Amyloidosis Foundation provides a list of amyloidosis treatment centers under “Patient Resources” on this website. These treatment centers offer the expertise and diagnostic accuracy with ALECT2 which are key factors for the patient. Once your diagnosis is confirmed, then a treatment plan can be laid out for your individual case. Depending on the symptoms, your local nephrologist (kidney doctor) and/or general physician should coordinate your care with the amyloidosis specialist to develop the best treatment program.
- AB2M
AB2M
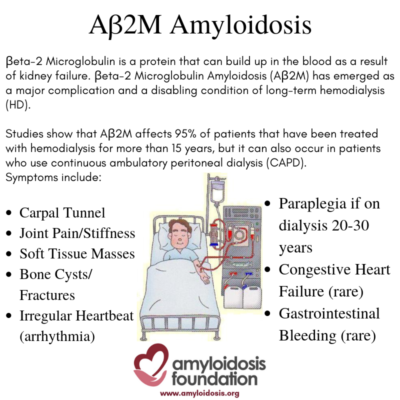
Healthy kidneys can clear out substances and toxins and remove them from the body. As kidney failure begins, the normal B2M protein increases. After many years of dialysis and with the ‘overexpression’ of this normal protein triggering misfolded amyloid proteins, the kidneys are unable to clear them out of the body, which then elevates the AB2M amyloid in the blood. As a result, the accumulation and deposit of this amyloid protein is often found in bones, joints and tendons.
What is B2M?
The B2M protein is normally found on the surface of many white blood cells and is present in small amounts in the blood, cerebral spinal fluid, and urine. With certain conditions and diseases, if the body produces an overabundance of these cells, then the body reacts by raising the level of the B2M protein. There may also be an increase of B2M protein if the body destroys these cells due to a disease.
The level of B2M does not diagnose a specific disease, but may help determine how advanced a disease is. The increased level of B2M in the body can occur with several diseases such as AB2M amyloidosis, multiple myeloma, lymphomas, or leukemia, among many others.
A lab test is performed that can analyze blood, urine, or fluid for increased levels of the B2M protein. Determining the level of B2M provides the doctor with more information about the health of the patient and the lab test is very helpful as a marker for detecting kidney damage, for distinguishing between different types of kidney disorders, or for tumors that occur with some blood cell cancers.
What are the symptoms of AB2M amyloidosis?
AB2M amyloid deposits can accumulate in bones and tissues (especially around the joints) because they are not excreted by the kidneys due to long-term kidney failure and dialysis.
Some studies suggest that carpal tunnel syndrome is the most common symptom. It is caused by pressure on the “median nerve” from amyloid deposits, resulting in tingling, numbness and pain in the fingers and wrist. Other common symptoms may include joint pain and/or stiffness (arthralgia) and soft tissue masses, causing tenderness and pain. In addition, bone cysts may occur (making hollow cavities in the bone) which in turn may lead to fractures.
Serious complications may arise, especially if the patient has been on dialysis for a very long time –longer than 20 to 30 years. For example, if ligaments and discs are severely damaged by the AB2M amyloid deposition, paraplegia is possible. In some cases, patients may develop cardiac (heart) symptoms, such as arrhythmia (irregular heartbeat), which can range from mild to severe.
Congestive heart failure is rare, but may occur, due to extreme amyloid deposition in the heart. In addition, GI (gastrointestinal) bleeding has been found in some patients, but this is considered a rare occurrence.How is AB2M amyloidosis diagnosed?
Like all the amyloidosis diseases, the diagnosis of amyloidosis is based on the evidence of amyloid deposits and a defined tissue or organ involvement.
It is recommended that the patient have typing done in a reference laboratory at an experienced amyloidosis center, and the typing should agree with the clinical features and symptoms of the patient’s disease. Unfortunately, costs of typing are not always covered by insurance, but it is very important to have correct typing before any treatment is initiated. A biopsy sample is taken from a suspected affected area, like the synovium (the smooth lining of a joint) or from a cystic bone lesion. Then this biopsy sample is sent to a lab for Congo-red staining. The lab will stain the biopsy sample and, if it turns an apple green color under a ‘polarizing’ microscope, then amyloidosis is confirmed. The lab will also define the type of the specific amyloid protein. Using an “immunostaining” technique, the sample is tested with the antibodies of different amyloid proteins. If this test shows the correct reaction to the B2M protein, then the diagnosis is AB2M amyloidosis.
In addition, there are various imaging tests that can support the diagnosis. A doctor may use x-rays, CT (computed tomography) scans, ultrasonography (ultrasound), and MRIs (magnetic resonance imaging) on the various joints, tendons, and bones that are affected by AB2M amyloidosis.
In some cases, joint erosion or cystic bone lesions or fractures may be observed before the AB2M diagnosis, resulting in the need to test for this amyloidosis disease.
What is the treatment for AB2M amyloidosis?
There is no treatment known for the disease itself. Progress has been observed when some patients use dialysis membranes that are known to have a higher clearance of Beta2-microglobulin microglobulin (B2M). Studies indicate that this may result in a delay in the onset of AB2M amyloidosis and the associated symptoms for these patients.
Kidneys rarely recover or improve when end stage renal failure has set in. For some patients a kidney transplant is an option, depending on the patient’s overall health, and can slow or stop the advancement of AB2M amyloidosis.
Other medical treatment consists of treating the symptoms and, if possible, surgically removing the amyloid deposits. For some symptoms, doctors prescribe corticosteroids and NSAIDs (non-steroidal anti-inflammatory drugs). Physical and occupational therapy may be helpful, in addition to wrist splints, cervical collars, and other braces and supports. If the patient develops cardiac or GI symptoms, a cardiologist or gastroenterologist should be consulted and individual treatments prescribed for that patient’s needs.
In addition, carpal tunnel release surgery can significantly reduce the pressure on the median nerve in the wrist, and surgery can be done to aid compression on the spinal cord to improve pain and discomfort. Other surgery on joints or tendons may be helpful to reduce pain and restore some function.
How common is AB2M amyloidosis?
Statistics vary on the occurrence of AB2M amyloidosis in the U.S. After a patient has been on dialysis for a long time period (more than 15 to 20 years) research data suggests an incidence of 95% or higher. In Europe, studies show the rate at 100% after 13 years. Most experts agree that the disease rarely occurs before 5 years of dialysis.
Many of these studies took place before the improvements in the efficiency of dialysis with the “high-flux dialysis” method, which may delay the development of AB2M amyloidosis. The likelihood of a patient developing AB2M amyloidosis varies with each individual. It all depends on the age of the patient, the age that the patient started on dialysis, the patient’s overall health, how long they have been on dialysis, and the type of dialysis membrane that the patient is using.
What kind of doctor should be consulted?
It is strongly recommended that you consult with a specialist in the field of amyloidosis. The Amyloidosis Foundation provides a list of amyloidosis treatment centers under “Patient Resources” on this website. These treatment centers offer the expertise and diagnostic accuracy with AB2M, which are key factors for the patient. Once your diagnosis is confirmed, then a treatment plan can be laid out for your individual case. Depending on the symptoms, your local nephrologist (kidney doctor) and/or general physician should coordinate your care with the amyloidosis specialist to develop the best treatment program.
- Cerebral Amyloid Angiopathy (CAA)
Cerebral Amyloid Angiopathy (CAA)
For more information click here
